Suppose two runners with low iron are taking iron supplements during their training. They follow similar workout routines and eat very similar diets. After several weeks, when race time comes, one runner suffers from low iron while the other has sufficient iron readings. Why would similar athletes have different health outcomes? It might well have to do with their DNA.
The completion of the Human Genome Project in 2003 and the International HapMap Project gave us a much better understanding of the relationship between DNA and nutrition. The goal of the HapMap Project is to compare the genetic sequences of different individuals to identify chromosomal regions where genetic variants are shared. And the Human Genome Project was the first complete mapping of all of the human genes. Both greatly advanced genetic medicine.
Mapping our DNA has opened up a world of opportunities for observing our ancestry, race, and ethnicity, and also for understanding the relationship between nutrition and our genes. This is called “nutrigenomics” and just like other areas of genetics, it is advancing rapidly.
One group of genes today’s nutrigenomic-based DNA tests can discover are levels of micronutrients. Approximately 40 micronutrients are required in the human diet. When a person doesn’t get enough of certain kinds, they are at risk for cancers, bone mass deficiencies, and neural tube defects.
Some of the important nutrients our bodies need and the foods they come from:
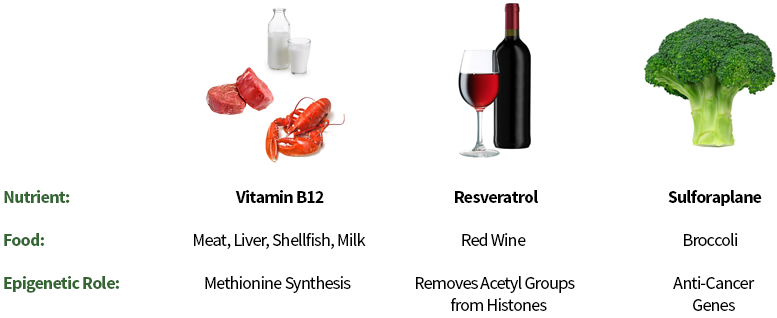
http://learn.genetics.utah.edu/content/epigenetics/nutrition
Another group uncovered are macronutrients — or your fats, carbohydrates, and proteins. If these are not in balance this has been shown linked to the initiation, development, and severity of chronic diseases like heart disease, cancer, and diabetes.
It is now understood that these nutrients (i.e., micronutrients, macronutrients) alter molecular processes such as DNA structure, gene expression, and metabolism, and these in turn, may alter disease initiation, development, or progression.
Nutrigenomic research focuses on the following 5 tenets: 1) common dietary chemicals act on the human genome, either directly or indirectly, to alter gene expression or structure; 2) under certain circumstances and in some individuals, diet can be a serious risk factor for a number of diseases; 3) some diet-regulated genes (and their normal, common variants) are likely to play a role in the onset, incidence, progression, and/or severity of chronic diseases; 4) the degree to which diet influences the balance between healthy and disease states may depend on an individual’s genetic makeup; and 5) dietary intervention based on knowledge of nutritional requirement, nutritional status, and genotype (i.e., “individualized nutrition”) can be used to prevent, mitigate, or cure chronic disease.
Yes, there is a connection between our DNA and what foods and nutrients we put into our bodies. What you eat counts! Since we are now understanding better which genetic variants are most important and precisely how they affect health, nutrigenomics is enabling us to make specific recommendations about what we eat.
According to World Health Organization, diet factors influence more than two-thirds of diseases. Nutrigenomics will encourage many changes in preventive medicine and major health problems will be those of preventable conditions associated with obesity, type-II diabetes, and cardiovascular disease. One of the keys to this will be fostering changes in lifestyle, principally nutrition and physical activity.


